Perspectives on Abnormal Behavior
2.4 Psychopharmacology
Susan Barron
Section Learning Objectives
- How do the majority of psychoactive drugs work in the brain?
- How does the route of administration affect how rewarding a drug might be?
- Why is grapefruit dangerous to consume with many psychotropic medications?
- Why might individualized drug doses based on genetic screening be helpful for treating conditions like depression?
- Why is there controversy regarding pharmacotherapy for children, adolescents, and the elderly?
Psychopharmacology is the study of how drugs affect behavior. If a drug changes your perception, or the way you feel or think, the drug exerts effects on your brain and nervous system. We call drugs that change the way you think or feel psychoactive or psychotropic drugs, and almost everyone has used a psychoactive drug at some point (yes, caffeine counts). Understanding some of the basics about psychopharmacology can help us better understand a wide range of things that interest psychologists and others. For example, the pharmacological treatment of certain neurodegenerative diseases such as Parkinson’s disease tells us something about the disease itself. The pharmacological treatments used to treat psychiatric conditions such as schizophrenia or depression have undergone amazing development since the 1950s, and the drugs used to treat these disorders tell us something about what is happening in the brain of individuals with these conditions. Finally, understanding something about the actions of drugs of abuse and their routes of administration can help us understand why some psychoactive drugs are so addictive. In this module, we will provide an overview of some of these topics as well as discuss some current controversial areas in the field of psychopharmacology.
Introduction
Psychopharmacology, the study of how drugs affect the brain and behavior, is a relatively new science, although people have probably been taking drugs to change how they feel from early in human history (consider the of eating fermented fruit, ancient beer recipes, chewing on the leaves of the cocaine plant for stimulant properties as just some examples). The word psychopharmacology itself tells us that this is a field that bridges our understanding of behavior (and brain) and pharmacology, and the range of topics included within this field is extremely broad.
Virtually any drug that changes the way you feel does this by altering how neurons communicate with each other. Neurons (more than 100 billion in your nervous system) communicate with each other by releasing a chemical (neurotransmitter) across a tiny space between two neurons (the synapse). When the neurotransmitter crosses the synapse, it binds to a postsynaptic receptor (protein) on the receiving neuron and the message may then be transmitted onward. Obviously, neurotransmission is far more complicated than this – links at the end of this module can provide some useful background if you want more detail – but the first step is understanding that virtually all psychoactive drugs interfere with or alter how neurons communicate with each other.
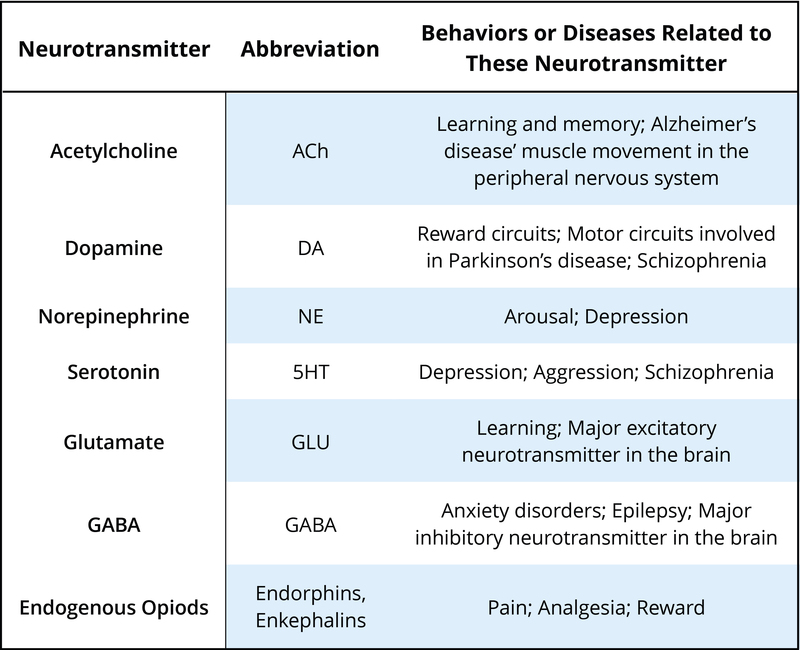
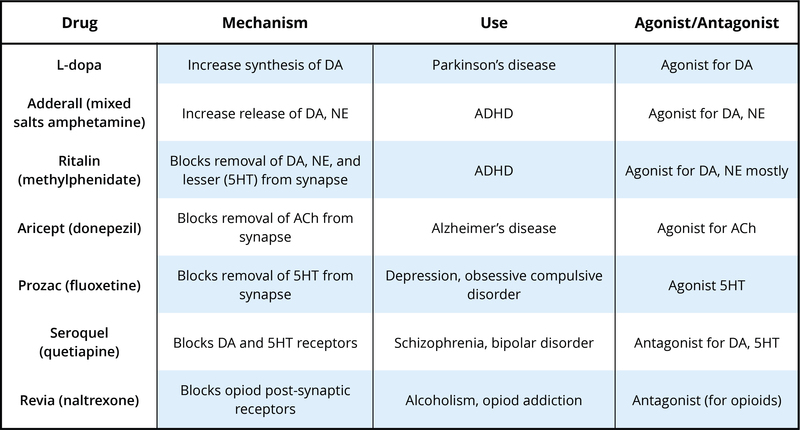
There are many neurotransmitters. Some of the most important in terms of psychopharmacological treatment and drugs of abuse are outlined in Table 1. The neurons that release these neurotransmitters, for the most part, are localized within specific circuits of the brain that mediate these behaviors. Psychoactive drugs can either increase activity at the synapse (these are called agonists) or reduce activity at the synapse (antagonists). Different drugs do this by different mechanisms, and some examples of agonists and antagonists are presented in Table 2. For each example, the drug’s trade name, which is the name of the drug provided by the drug company, and generic name (in parentheses) are provided.
A very useful link at the end of this module shows the various steps involved in neurotransmission and some ways drugs can alter this.
Table 2 provides examples of drugs and their primary mechanism of action, but it is very important to realize that drugs also have effects on other neurotransmitters. This contributes to the kinds of side effects that are observed when someone takes a particular drug. The reality is that no drugs currently available work only exactly where we would like in the brain or only on a specific neurotransmitter. In many cases, individuals are sometimes prescribed one psychotropic drug but then may also have to take additional drugs to reduce the side effects caused by the initial drug. Sometimes individuals stop taking medication because the side effects can be so profound.
Pharmacokinetics: What Is It – Why Is It Important?
While this section may sound more like pharmacology, it is important to realize how important pharmacokinetics can be when considering psychoactive drugs. Pharmacokinetics refers to how the body handles a drug that we take. As mentioned earlier, psychoactive drugs exert their effects on behavior by altering neuronal communication in the brain, and the majority of drugs reach the brain by traveling in the blood. The acronym ADME is often used with A standing for absorption (how the drug gets into the blood), Distribution (how the drug gets to the organ of interest – in this module, that is the brain), Metabolism (how the drug is broken down so it no longer exerts its psychoactive effects), and Excretion (how the drug leaves the body). We will talk about a couple of these to show their importance for considering psychoactive drugs.
Drug Administration
There are many ways to take drugs, and these routes of drug administration can have a significant impact on how quickly that drug reaches brain. The most common route of administration is oral administration, which is relatively slow and – perhaps surprisingly – often the most variable and complex route of administration. Drugs enter the stomach and then get absorbed by the blood supply and capillaries that line the small intestine. The rate of absorption can be affected by a variety of factors including the quantity and the type of food in the stomach (e.g., fats vs. proteins). This is why the medicine label for some drugs (like antibiotics) may specifically state foods that you should or should NOT consume within an hour of taking the drug because they can affect the rate of absorption. Two of the most rapid routes of administration include inhalation (i.e., smoking or gaseous anesthesia) and intravenous (IV) in which the drug is injected directly into the vein and hence the blood supply. Both of these routes of administration can get the drug to brain in less than 10 seconds. IV administration also has the distinction of being the most dangerous because if there is an adverse drug reaction, there is very little time to administer any antidote, as in the case of an IV heroin overdose.
Why might how quickly a drug gets to the brain be important? If a drug activates the reward circuits in the brain AND it reaches the brain very quickly, the drug has a high risk for abuse and addiction. Psychostimulants like amphetamine or cocaine are examples of drugs that have high risk for abuse because they are agonists at DA neurons involved in reward AND because these drugs exist in forms that can be either smoked or injected intravenously. Some argue that cigarette smoking is one of the hardest addictions to quit, and although part of the reason for this may be that smoking gets the nicotine into the brain very quickly (and indirectly acts on DA neurons), it is a more complicated story. For drugs that reach the brain very quickly, not only is the drug very addictive, but so are the cues associated with the drug (see Rohsenow, Niaura, Childress, Abrams, & Monti, 1990). For a crack user, this could be the pipe that they use to smoke the drug. For a cigarette smoker, however, it could be something as normal as finishing dinner or waking up in the morning (if that is when the smoker usually has a cigarette). For both the crack user and the cigarette smoker, the cues associated with the drug may actually cause craving that is alleviated by (you guessed it) – lighting a cigarette or using crack (i.e., relapse). This is one of the reasons individuals that enroll in drug treatment programs, especially out-of-town programs, are at significant risk of relapse if they later find themselves in proximity to old haunts, friends, etc. But this is much more difficult for a cigarette smoker. How can someone avoid eating? Or avoid waking up in the morning, etc. These examples help you begin to understand how important the route of administration can be for psychoactive drugs.
Drug Metabolism
Metabolism involves the breakdown of psychoactive drugs, and this occurs primarily in the liver. The liver produces enzymes (proteins that speed up a chemical reaction), and these enzymes help catalyze a chemical reaction that breaks down psychoactive drugs. Enzymes exist in “families,” and many psychoactive drugs are broken down by the same family of enzymes, the cytochrome P450 superfamily. There is not a unique enzyme for each drug; rather, certain enzymes can break down a wide variety of drugs. Tolerance to the effects of many drugs can occur with repeated exposure; that is, the drug produces less of an effect over time, so more of the drug is needed to get the same effect. This is particularly true for sedative drugs like alcohol or opiate-based painkillers. Metabolic tolerance is one kind of tolerance and it takes place in the liver. Some drugs (like alcohol) cause enzyme induction – an increase in the enzymes produced by the liver. For example, chronic drinking results in alcohol being broken down more quickly, so the alcoholic needs to drink more to get the same effect – of course, until so much alcohol is consumed that it damages the liver (alcohol can cause fatty liver or cirrhosis).
Recent Issues Related to Psychotropic Drugs and Metabolism
Grapefruit Juice and Metabolism

Certain types of food in the stomach can alter the rate of drug absorption, and other foods can also alter the rate of drug metabolism. The most well known is grapefruit juice. Grapefruit juice suppresses cytochrome P450 enzymes in the liver, and these liver enzymes normally break down a large variety of drugs (including some of the psychotropic drugs). If the enzymes are suppressed, drug levels can build up to potentially toxic levels. In this case, the effects can persist for extended periods of time after the consumption of grapefruit juice. As of 2013, there are at least 85 drugs shown to adversely interact with grapefruit juice (Bailey, Dresser, & Arnold, 2013). Some psychotropic drugs that are likely to interact with grapefruit juice include carbamazepine (Tegretol), prescribed for bipolar disorder; diazepam (Valium), used to treat anxiety, alcohol withdrawal, and muscle spasms; and fluvoxamine (Luvox), used to treat obsessive compulsive disorder and depression. A link at the end of this module gives the latest list of drugs reported to have this unusual interaction.
Individualized Therapy, Metabolic Differences, and Potential Prescribing Approaches for the Future
Mental illnesses contribute to more disability in western countries than all other illnesses including cancer and heart disease. Depression alone is predicted to be the second largest contributor to disease burden by 2020 (World Health Organization, 2004). The numbers of people affected by mental health issues are pretty astonishing, with estimates that 25% of adults experience a mental health issue in any given year, and this affects not only the individual but their friends and family. One in 17 adults experiences a serious mental illness (Kessler, Chiu, Demler, & Walters, 2005). Newer antidepressants are probably the most frequently prescribed drugs for treating mental health issues, although there is no “magic bullet” for treating depression or other conditions. Pharmacotherapy with psychological therapy may be the most beneficial treatment approach for many psychiatric conditions, but there are still many unanswered questions. For example, why does one antidepressant help one individual yet have no effect for another? Antidepressants can take 4 to 6 weeks to start improving depressive symptoms, and we don’t really understand why. Many people do not respond to the first antidepressant prescribed and may have to try different drugs before finding something that works for them. Other people just do not improve with antidepressants (Ioannidis, 2008). As we better understand why individuals differ, the easier and more rapidly we will be able to help people in distress.
One area that has received interest recently has to do with an individualized treatment approach. We now know that there are genetic differences in some of the cytochrome P450 enzymes and their ability to break down drugs. The general population falls into the following 4 categories: 1) ultra-extensive metabolizers break down certain drugs (like some of the current antidepressants) very, very quickly, 2) extensive metabolizers are also able to break down drugs fairly quickly, 3) intermediate metabolizers break down drugs more slowly than either of the two above groups, and finally 4) poor metabolizers break down drugs much more slowly than all of the other groups. Now consider someone receiving a prescription for an antidepressant – what would the consequences be if they were either an ultra-extensive metabolizer or a poor metabolizer? The ultra-extensive metabolizer would be given antidepressants and told it will probably take 4 to 6 weeks to begin working (this is true), but they metabolize the medication so quickly that it will never be effective for them. In contrast, the poor metabolizer given the same daily dose of the same antidepressant may build up such high levels in their blood (because they are not breaking the drug down), that they will have a wide range of side effects and feel really badly – also not a positive outcome. What if – instead – prior to prescribing an antidepressant, the doctor could take a blood sample and determine which type of metabolizer a patient actually was? They could then make a much more informed decision about the best dose to prescribe. There are new genetic tests now available to better individualize treatment in just this way. A blood sample can determine (at least for some drugs) which category an individual fits into, but we need data to determine if this actually is effective for treating depression or other mental illnesses (Zhou, 2009). Currently, this genetic test is expensive and not many health insurance plans cover this screen, but this may be an important component in the future of psychopharmacology.
Other Controversial Issues
Juveniles and Psychopharmacology
A recent Centers for Disease Control (CDC) report has suggested that as many as 1 in 5 children between the ages of 5 and 17 may have some type of mental disorder (e.g., ADHD, autism, anxiety, depression) (CDC, 2013). The incidence of bipolar disorder in children and adolescents has also increased 40 times in the past decade (Moreno, Laje, Blanco, Jiang, Schmidt, & Olfson, 2007), and it is now estimated that 1 in 88 children have been diagnosed with an autism spectrum disorder (CDC, 2011). Why has there been such an increase in these numbers? There is no single answer to this important question. Some believe that greater public awareness has contributed to increased teacher and parent referrals. Others argue that the increase stems from changes in criterion currently used for diagnosing. Still others suggest environmental factors, either prenatally or postnatally, have contributed to this upsurge.

We do not have an answer, but the question does bring up an additional controversy related to how we should treat this population of children and adolescents. Many psychotropic drugs used for treating psychiatric disorders have been tested in adults, but few have been tested for safety or efficacy with children or adolescents. The most well-established psychotropics prescribed for children and adolescents are the psychostimulant drugs used for treating attention deficit hyperactivity disorder (ADHD), and there are clinical data on how effective these drugs are. However, we know far less about the safety and efficacy in young populations of the drugs typically prescribed for treating anxiety, depression, or other psychiatric disorders. The young brain continues to mature until probably well after age 20, so some scientists are concerned that drugs that alter neuronal activity in the developing brain could have significant consequences. There is an obvious need for clinical trials in children and adolescents to test the safety and effectiveness of many of these drugs, which also brings up a variety of ethical questions about who decides what children and adolescents will participate in these clinical trials, who can give consent, who receives reimbursements, etc.
The Elderly and Psychopharmacology
Another population that has not typically been included in clinical trials to determine the safety or effectiveness of psychotropic drugs is the elderly. Currently, there is very little high-quality evidence to guide prescribing for older people – clinical trials often exclude people with multiple comorbidities (other diseases, conditions, etc.), which are typical for elderly populations (see Hilmer and Gnjidict, 2008; Pollock, Forsyth, & Bies, 2008). This is a serious issue because the elderly consume a disproportionate number of the prescription meds prescribed. The term polypharmacy refers to the use of multiple drugs, which is very common in elderly populations in the United States. As our population ages, some estimate that the proportion of people 65 or older will reach 20% of the U.S. population by 2030, with this group consuming 40% of the prescribed medications. As shown in Table 3 (from Schwartz and Abernethy, 2008), it is quite clear why the typical clinical trial that looks at the safety and effectiveness of psychotropic drugs can be problematic if we try to interpret these results for an elderly population.
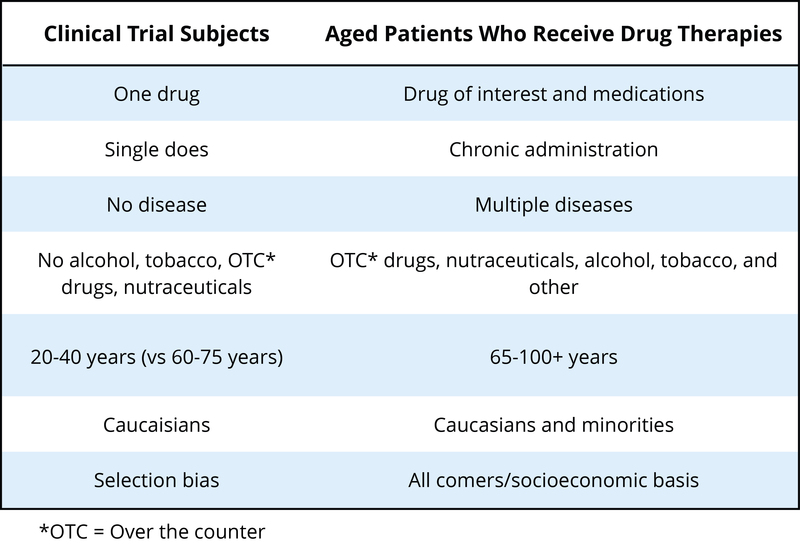
Metabolism of drugs is often slowed considerably for elderly populations, so less drug can produce the same effect (or all too often, too much drug can result in a variety of side effects). One of the greatest risk factors for elderly populations is falling (and breaking bones), which can happen if the elderly person gets dizzy from too much of a drug. There is also evidence that psychotropic medications can reduce bone density (thus worsening the consequences if someone falls) (Brown & Mezuk, 2012). Although we are gaining an awareness about some of the issues facing pharmacotherapy in older populations, this is a very complex area with many medical and ethical questions.
This module provided an introduction of some of the important areas in the field of psychopharmacology. It should be apparent that this module just touched on a number of topics included in this field. It should also be apparent that understanding more about psychopharmacology is important to anyone interested in understanding behavior and that our understanding of issues in this field has important implications for society.
Outside Resources
Video: Neurotransmission
Web: Description of how some drugs work and the brain areas involved – 1 http://www.drugabuse.gov/news-events/nida-notes/2007/10/impacts-drugs-neurotransmission
Web: Description of how some drugs work and the brain areas involved – 2 http://learn.genetics.utah.edu/content/addiction/mouse/
Web: Information about how neurons communicate and the reward pathways http://learn.genetics.utah.edu/content/addiction/rewardbehavior/
Web: National Institute of Alcohol Abuse and Alcoholism http://www.niaaa.nih.gov/
Web: National Institute of Drug Abuse http://www.drugabuse.gov/
Web: National Institute of Mental Healthhttp://www.nimh.nih.gov/index.shtml
Web: Neurotransmission https://science.education.nih.gov/supplements/nih2/Addiction/activities/lesson2_neurotransmission.html
Web: Report of the Working Group on Psychotropic Medications for Children and Adolescents: Psychopharmacological, Psychosocial, and Combined Interventions for Childhood Disorders: Evidence Base, Contextual Factors, and Future Directions (2008): http://www.apa.org/pi/families/resources/child-medications.pdf
Web: Ways drugs can alter neurotransmission http://thebrain.mcgill.ca/flash/d/d_03/d_03_m/d_03_m_par/d_03_m_par.html
Discussion Questions
- What are some of the issues surrounding prescribing medications for children and adolescents? How might this be improved?
- What are some of the factors that can affect relapse to an addictive drug?
- How might prescribing medications for depression be improved in the future to increase the likelihood that a drug would work and minimize side effects?
References
Bailey D. G., Dresser G., & Arnold J. M. (2013). Grapefruit-medication interactions: forbidden fruit or avoidable consequences? Canadian Medical Association Journal, 185, 309–316.
Barron, S. (2020). Psychopharmacology. In R. Biswas-Diener & E. Diener (Eds), Noba textbook series: Psychology. Champaign, IL: DEF publishers. Retrieved from http://noba.to/umx6f2t8.
Brown, M. J., & Mezuk, B. (2012). Brains, bones, and aging: psychotropic medications and bone health among older adults. Current Osteoporosis Reports, 10, 303–311.
Centers for Disease Control and Prevention (2011) Prevalence of autism spectrum disorders – autism and developmental disabilities monitoring network, 14 sites, United States, 2008. Morbidity and Mortality Weekly Report 61(SS03) 1–19.
Centers for Disease Control and Prevention. (2013) Mental health surveillance among children – United States, 2005—2011. Morbidity and Mortality Weekly Report 62 Suppl, 1-35.
Hilmer, N., & Gnjidict, D. (2008). The effects of polypharmacy in older adults. Clinical Pharmacology & Therapeutics, 85, 86–88.
Ioannidis, J. P. A. (2008). Effectiveness of antidepressants: an evidence myth constructed from a thousand randomized trials? Philosophy, Ethics and Humanities in Medicine, 3,14.
Kessler, R. C., Chiu, W. T., Demler, O., & Walters, E. E. (2005). Prevalence, severity, and comorbidity of twelve-month DSM-IV disorders in the National Comorbidity Survey Replication (NCS-R). Archives of General Psychiatry, 62, 617–627.
Moreno, C., Laje, G., Blanco, C., Jiang, H., Schmidt, A. B., & Olfson, M., (2007). National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Archives of General Psychiatry, 64(9), 1032–1039.
Pollock, B. G., Forsyth, C. E., & Bies, R. R. (2008). The critical role of clinical pharmacology in geriatric psychopharmacology. Clinical Pharmacology & Therapeutics, 85, 89–93.
Rohsenow, D. J., Niaura, R. S., Childress, A. R., Abrams, D. B., &, Monti, P. M. (1990). Cue reactivity in addictive behaviors: Theoretical and treatment implications. International Journal of Addiction, 25, 957–993.
Schwartz, J. B., & Abernethy, D. R. (2008). Aging and medications: Past, present, future. Clinical Pharmacology & Therapeutics, 85, 3–10.
World Health Organization. (2004). Promoting mental health: concepts, emerging evidence, practice (Summary Report). Geneva, Switzerland: Author. Retrieved from http://www.who.int/mental_health/evidence/en/promoting_mhh.pdf
Zhou, S. F. (2009). Polymorphism of human cytochrome P450 2D6 and its clinical significance: Part II. Clinical Pharmacokinetics, 48, 761–804.

