Schizophrenia & Related Psychotic Disorders
5.1 Schizophrenia & Related Psychotic Disorders
Deanna M. Barch and Jorden A. Cummings
Section Learning Objectives
- Describe the signs and symptoms of schizophrenia and related psychotic disorders.
- Describe the most well-replicated cognitive and neurobiological changes associated with schizophrenia.
- Describe the potential risk factors for the development of schizophrenia.
- Describe the controversies associated with “clinical high risk” approaches to identifying individuals at risk for the development of schizophrenia.
- Describe the treatments that work for some of the symptoms of schizophrenia.
The Phenomenology of Schizophrenia and Related Psychotic Disorders
Most of you have probably had the experience of walking down the street in a city and seeing a person you thought was acting oddly. They may have been dressed in an unusual way, perhaps disheveled or wearing an unusual collection of clothes, makeup, or jewelry that did not seem to fit any particular group or subculture. They may have been talking to themselves or yelling at someone you could not see. If you tried to speak to them, they may have been difficult to follow or understand, or they may have acted paranoid or started telling a bizarre story about the people who were plotting against them. If so, chances are that you have encountered an individual with schizophrenia or another type of psychotic disorder. If you have watched the movie A Beautiful Mind or The Fisher King, you have also seen a portrayal of someone thought to have schizophrenia. Sadly, a few of the individuals who have committed some of the recently highly publicized mass murders may have had schizophrenia, though most people who commit such crimes do not have schizophrenia and the vast majority of people with schizophrenia are not dangerous. It is also likely that you have met people with schizophrenia without ever knowing it, as they may suffer in silence or stay isolated to protect themselves from the horrors they see, hear, or believe are operating in the outside world. As these examples begin to illustrate, psychotic disorders involve many different types of symptoms, including delusions, hallucinations, disorganized speech and behavior, abnormal motor behavior (including catatonia), and negative symptoms such anhedonia/amotivation and blunted affect/reduced speech.
Delusions are false beliefs that are often fixed, hard to change even when the person is presented with conflicting information, and are often culturally influenced in their content (e.g., delusions involving Jesus in Judeo-Christian cultures, delusions involving Allah in Muslim cultures). They can be terrifying for the person, who may remain convinced that they are true even when loved ones and friends present them with clear information that they cannot be true. There are many different types or themes to delusions.

The most common delusions are persecutory and involve the belief that individuals or groups are trying to hurt, harm, or plot against the person in some way. These can be people that the person knows (people at work, the neighbors, family members), or more abstract groups (the FBI, the CIA, aliens, etc.). Other types of delusions include grandiose delusions, where the person believes that they have some special power or ability (e.g., I am the new Buddha, I am a rock star); referential delusions, where the person believes that events or objects in the environment have special meaning for them (e.g., that song on the radio is being played specifically for me); or other types of delusions where the person may believe that others are controlling their thoughts and actions, their thoughts are being broadcast aloud, or that others can read their mind (or they can read other people’s minds).
When you see a person on the street talking to themselves or shouting at other people, they are experiencing hallucinations. These are perceptual experiences that occur even when there is no stimulus in the outside world generating the experiences. They can be auditory, visual, olfactory (smell), gustatory (taste), or somatic (touch). The most common hallucinations in psychosis (at least in adults) are auditory, and can involve one or more voices talking about the person, commenting on the person’s behavior, or giving them orders. The content of the hallucinations is frequently negative (“you are a loser,” “that drawing is stupid,” “you should go kill yourself”) and can be the voice of someone the person knows or a complete stranger. Sometimes the voices sound as if they are coming from outside the person’s head. Other times the voices seem to be coming from inside the person’s head, but are not experienced the same as the person’s inner thoughts or inner speech.

Talking to someone with schizophrenia is sometimes difficult, as their speech may be difficult to follow, either because their answers do not clearly flow from your questions, or because one sentence does not logically follow from another. This is referred to as disorganized speech, and it can be present even when the person is writing. Disorganized behavior can include odd dress, odd makeup (e.g., lipstick outlining a mouth for 1 inch), or unusual rituals (e.g., repetitive hand gestures). Abnormal motor behavior can include catatonia, which refers to a variety of behaviors that seem to reflect a reduction in responsiveness to the external environment. This can include holding unusual postures for long periods of time, failing to respond to verbal or motor prompts from another person, or excessive and seemingly purposeless motor activity.
Some of the most debilitating symptoms of schizophrenia are difficult for others to see. These include what people refer to as “negative symptoms” or the absence of certain things we typically expect most people to have. For example, anhedonia or amotivation reflect a lack of apparent interest in or drive to engage in social or recreational activities. These symptoms can manifest as a great amount of time spent in physical immobility. Importantly, anhedonia and amotivation do not seem to reflect a lack of enjoyment in pleasurable activities or events (Cohen & Minor, 2010; Kring & Moran, 2008; Llerena, Strauss, & Cohen, 2012) but rather a reduced drive or ability to take the steps necessary to obtain the potentially positive outcomes (Barch & Dowd, 2010). Flat affect and reduced speech (alogia) reflect a lack of showing emotions through facial expressions, gestures, and speech intonation, as well as a reduced amount of speech and increased pause frequency and duration.
In many ways, the types of symptoms associated with psychosis are the most difficult for us to understand, as they may seem far outside the range of our normal experiences. Unlike depression or anxiety, many of us may not have had experiences that we think of as on the same continuum as psychosis. However, just like many of the other forms of psychopathology described in this book, the types of psychotic symptoms that characterize disorders like schizophrenia are on a continuum with “normal” mental experiences. For example, work by Jim van Os in the Netherlands has shown that a surprisingly large percentage of the general population (10%+) experience psychotic-like symptoms, though many fewer have multiple experiences and most will not continue to experience these symptoms in the long run (Verdoux & van Os, 2002). Similarly, work in a general population of adolescents and young adults in Kenya has also shown that a relatively high percentage of individuals experience one or more psychotic-like experiences (~19%) at some point in their lives (Mamah et al., 2012; Ndetei et al., 2012), though again most will not go on to develop a full-blown psychotic disorder.
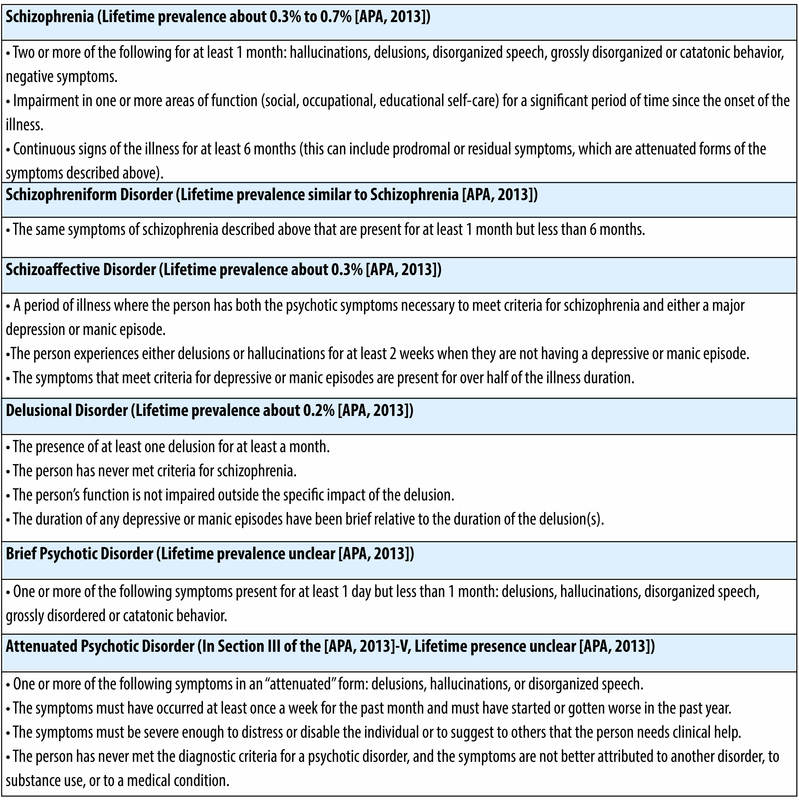
Schizophrenia is the primary disorder that comes to mind when we discuss “psychotic” disorders (see Table 1 for diagnostic criteria), though there are a number of other disorders that share one or more features with schizophrenia. In the remainder of this module, we will use the terms “psychosis” and “schizophrenia” somewhat interchangeably, given that most of the research has focused on schizophrenia. In addition to schizophrenia (see Table 1), other psychotic disorders include schizophreniform disorder (a briefer version of schizophrenia), schizoaffective disorder (a mixture of psychosis and depression/mania symptoms), delusional disorder (the experience of only delusions), and brief psychotic disorder (psychotic symptoms that last only a few days or weeks).
The Cognitive Neuroscience of Schizophrenia
As described above, when we think of the core symptoms of psychotic disorders such as schizophrenia, we think of people who hear voices, see visions, and have false beliefs about reality (i.e., delusions). However, problems in cognitive function are also a critical aspect of psychotic disorders and of schizophrenia in particular. This emphasis on cognition in schizophrenia is in part due to the growing body of research suggesting that cognitive problems in schizophrenia are a major source of disability and loss of functional capacity (Green, 2006; Nuechterlein et al., 2011). The cognitive deficits that are present in schizophrenia are widespread and can include problems with episodic memory (the ability to learn and retrieve new information or episodes in one’s life), working memory (the ability to maintain information over a short period of time, such as 30 seconds), and other tasks that require one to “control” or regulate one’s behavior (Barch & Ceaser, 2012; Bora, Yucel, & Pantelis, 2009a; Fioravanti, Carlone, Vitale, Cinti, & Clare, 2005; Forbes, Carrick, McIntosh, & Lawrie, 2009; Mesholam-Gately, Giuliano, Goff, Faraone, & Seidman, 2009). Individuals with schizophrenia also have difficulty with what is referred to as “processing speed” and are frequently slower than healthy individuals on almost all tasks. Importantly, these cognitive deficits are present prior to the onset of the illness (Fusar-Poli et al., 2007) and are also present, albeit in a milder form, in the first-degree relatives of people with schizophrenia (Snitz, Macdonald, & Carter, 2006). This suggests that cognitive impairments in schizophrenia reflect part of the risk for the development of psychosis, rather than being an outcome of developing psychosis. Further, people with schizophrenia who have more severe cognitive problems also tend to have more severe negative symptoms and more disorganized speech and behavior (Barch, Carter, & Cohen, 2003; Barch et al., 1999; Dominguez Mde, Viechtbauer, Simons, van Os, & Krabbendam, 2009; Ventura, Hellemann, Thames, Koellner, & Nuechterlein, 2009; Ventura, Thames, Wood, Guzik, & Hellemann, 2010). In addition, people with more cognitive problems have worse function in everyday life (Bowie et al., 2008; Bowie, Reichenberg, Patterson, Heaton, & Harvey, 2006; Fett et al., 2011).

Some people with schizophrenia also show deficits in what is referred to as social cognition, though it is not clear whether such problems are separate from the cognitive problems described above or the result of them (Hoe, Nakagami, Green, & Brekke, 2012; Kerr & Neale, 1993; van Hooren et al., 2008). This includes problems with the recognition of emotional expressions on the faces of other individuals (Kohler, Walker, Martin, Healey, & Moberg, 2010) and problems inferring the intentions of other people (theory of mind) (Bora, Yucel, & Pantelis, 2009b). Individuals with schizophrenia who have more problems with social cognition also tend to have more negative and disorganized symptoms (Ventura, Wood, & Hellemann, 2011), as well as worse community function (Fett et al., 2011).
The advent of neuroimaging techniques such as structural and functional magnetic resonance imaging and positron emission tomography opened up the ability to try to understand the brain mechanisms of the symptoms of schizophrenia as well as the cognitive impairments found in psychosis. For example, a number of studies have suggested that delusions in psychosis may be associated with problems in “salience” detection mechanisms supported by the ventral striatum (Jensen & Kapur, 2009; Jensen et al., 2008; Kapur, 2003; Kapur, Mizrahi, & Li, 2005; Murray et al., 2008) and the anterior prefrontal cortex (Corlett et al., 2006; Corlett, Honey, & Fletcher, 2007; Corlett, Murray, et al., 2007a, 2007b). These are regions of the brain that normally increase their activity when something important (aka “salient”) happens in the environment. If these brain regions misfire, it may lead individuals with psychosis to mistakenly attribute importance to irrelevant or unconnected events. Further, there is good evidence that problems in working memory and cognitive control in schizophrenia are related to problems in the function of a region of the brain called the dorsolateral prefrontal cortex (DLPFC) (Minzenberg, Laird, Thelen, Carter, & Glahn, 2009; Ragland et al., 2009). These problems include changes in how the DLPFC works when people are doing working-memory or cognitive-control tasks, and problems with how this brain region is connected to other brain regions important for working memory and cognitive control, including the posterior parietal cortex (e.g., Karlsgodt et al., 2008; Kim et al., 2003; Schlosser et al., 2003), the anterior cingulate (Repovs & Barch, 2012), and temporal cortex (e.g., Fletcher et al., 1995; Meyer-Lindenberg et al., 2001). In terms of understanding episodic memory problems in schizophrenia, many researchers have focused on medial temporal lobe deficits, with a specific focus on the hippocampus (e.g., Heckers & Konradi, 2010). This is because there is much data from humans and animals showing that the hippocampus is important for the creation of new memories (Squire, 1992). However, it has become increasingly clear that problems with the DLPFC also make important contributions to episodic memory deficits in schizophrenia (Ragland et al., 2009), probably because this part of the brain is important for controlling our use of memory.
In addition to problems with regions such as the DLFPC and medial temporal lobes in schizophrenia described above, magnetic resonance neuroimaging studies have also identified changes in cellular architecture, white matter connectivity, and gray matter volume in a variety of regions that include the prefrontal and temporal cortices (Bora et al., 2011). People with schizophrenia also show reduced overall brain volume, and reductions in brain volume as people get older may be larger in those with schizophrenia than in healthy people (Olabi et al., 2011). Taking antipsychotic medications or taking drugs such as marijuana, alcohol, and tobacco may cause some of these structural changes. However, these structural changes are not completely explained by medications or substance use alone. Further, both functional and structural brain changes are seen, again to a milder degree, in the first-degree relatives of people with schizophrenia (Boos, Aleman, Cahn, Pol, & Kahn, 2007; Brans et al., 2008; Fusar-Poli et al., 2007; MacDonald, Thermenos, Barch, & Seidman, 2009). This again suggests that that neural changes associated with schizophrenia are related to a genetic risk for this illness.
Risk Factors for Developing Schizophrenia
It is clear that there are important genetic contributions to the likelihood that someone will develop schizophrenia, with consistent evidence from family, twin, and adoption studies. (Sullivan, Kendler, & Neale, 2003). However, there is no “schizophrenia gene” and it is likely that the genetic risk for schizophrenia reflects the summation of many different genes that each contribute something to the likelihood of developing psychosis (Gottesman & Shields, 1967; Owen, Craddock, & O’Donovan, 2010). Further, schizophrenia is a very heterogeneous disorder, which means that two different people with “schizophrenia” may each have very different symptoms (e.g., one has hallucinations and delusions, the other has disorganized speech and negative symptoms). This makes it even more challenging to identify specific genes associated with risk for psychosis. Importantly, many studies also now suggest that at least some of the genes potentially associated with schizophrenia are also associated with other mental health conditions, including bipolar disorder, depression, and autism (Gejman, Sanders, & Kendler, 2011; Kim, Zerwas, Trace, & Sullivan, 2011; Owen et al., 2010; Rutter, Kim-Cohen, & Maughan, 2006).

There are also a number of environmental factors that are associated with an increased risk of developing schizophrenia. For example, problems during pregnancy such as increased stress, infection, malnutrition, and/or diabetes have been associated with increased risk of schizophrenia. In addition, complications that occur at the time of birth and which cause hypoxia (lack of oxygen) are also associated with an increased risk for developing schizophrenia (Cannon, Jones, & Murray, 2002; Miller et al., 2011). Children born to older fathers are also at a somewhat increased risk of developing schizophrenia. Further, using cannabis increases risk for developing psychosis, especially if you have other risk factors (Casadio, Fernandes, Murray, & Di Forti, 2011; Luzi, Morrison, Powell, di Forti, & Murray, 2008). The likelihood of developing schizophrenia is also higher for kids who grow up in urban settings (March et al., 2008) and for some minority ethnic groups (Bourque, van der Ven, & Malla, 2011). Both of these factors may reflect higher social and environmental stress in these settings. Unfortunately, none of these risk factors is specific enough to be particularly useful in a clinical setting, and most people with these “risk” factors do not develop schizophrenia. However, together they are beginning to give us clues as the neurodevelopmental factors that may lead someone to be at an increased risk for developing this disease.
An important research area on risk for psychosis has been work with individuals who may be at “clinical high risk.” These are individuals who are showing attenuated (milder) symptoms of psychosis that have developed recently and who are experiencing some distress or disability associated with these symptoms. When people with these types of symptoms are followed over time, about 35% of them develop a psychotic disorder (Cannon et al., 2008), most frequently schizophrenia (Fusar-Poli, McGuire, & Borgwardt, 2012). In order to identify these individuals, a new category of diagnosis, called “Attenuated Psychotic Syndrome,” was added to Section III (the section for disorders in need of further study) of the DSM-5 (see Table 1 for symptoms) (APA, 2013). However, adding this diagnostic category to the DSM-5 created a good deal of controversy (Batstra & Frances, 2012; Fusar-Poli & Yung, 2012). Many scientists and clinicians have been worried that including “risk” states in the DSM-5 would create mental disorders where none exist, that these individuals are often already seeking treatment for other problems, and that it is not clear that we have good treatments to stop these individuals from developing to psychosis. However, the counterarguments have been that there is evidence that individuals with high-risk symptoms develop psychosis at a much higher rate than individuals with other types of psychiatric symptoms, and that the inclusion of Attenuated Psychotic Syndrome in Section III will spur important research that might have clinical benefits. Further, there is some evidence that non-invasive treatments such as omega-3 fatty acids and intensive family intervention may help reduce the development of full-blown psychosis (Preti & Cella, 2010) in people who have high-risk symptoms.
Treatment of Schizophrenia
The currently available treatments for schizophrenia leave much to be desired, and the search for more effective treatments for both the psychotic symptoms of schizophrenia (e.g., hallucinations and delusions) as well as cognitive deficits and negative symptoms is a highly active area of research. The first line of treatment for schizophrenia and other psychotic disorders is the use of antipsychotic medications. There are two primary types of antipsychotic medications, referred to as “typical” and “atypical.” The fact that “typical” antipsychotics helped some symptoms of schizophrenia was discovered serendipitously more than 60 years ago (Carpenter & Davis, 2012; Lopez-Munoz et al., 2005). These are drugs that all share a common feature of being a strong block of the D2 type dopamine receptor. Although these drugs can help reduce hallucinations, delusions, and disorganized speech, they do little to improve cognitive deficits or negative symptoms and can be associated with distressing motor side effects. The newer generation of antipsychotics is referred to as “atypical” antipsychotics. These drugs have more mixed mechanisms of action in terms of the receptor types that they influence, though most of them also influence D2 receptors. These newer antipsychotics are not necessarily more helpful for schizophrenia but have fewer motor side effects. However, many of the atypical antipsychotics are associated with side effects referred to as the “metabolic syndrome,” which includes weight gain and increased risk for cardiovascular illness, Type-2 diabetes, and mortality (Lieberman et al., 2005).
The evidence that cognitive deficits also contribute to functional impairment in schizophrenia has led to an increased search for treatments that might enhance cognitive function in schizophrenia. Unfortunately, as of yet, there are no pharmacological treatments that work consistently to improve cognition in schizophrenia, though many new types of drugs are currently under exploration. However, there is a type of psychological intervention, referred to as cognitive remediation, which has shown some evidence of helping cognition and function in schizophrenia. In particular, a version of this treatment called Cognitive Enhancement Therapy (CET) has been shown to improve cognition, functional outcome, social cognition, and to protect against gray matter loss (Eack et al., 2009; Eack, Greenwald, Hogarty, & Keshavan, 2010; Eack et al., 2010; Eack, Pogue-Geile, Greenwald, Hogarty, & Keshavan, 2010; Hogarty, Greenwald, & Eack, 2006) in young individuals with schizophrenia. The development of new treatments such as Cognitive Enhancement Therapy provides some hope that we will be able to develop new and better approaches to improving the lives of individuals with this serious mental health condition and potentially even prevent it some day.
Outside Resources
Book: Ben Behind His Voices: One family’s journal from the chaos of schizophrenia to hope (2011). Randye Kaye. Rowman and Littlefield.
Book: Conquering Schizophrenia: A father, his son, and a medical breakthrough (1997). Peter Wyden. Knopf.
Book: Henry’s Demons: Living with schizophrenia, a father and son’s story (2011). Henry and Patrick Cockburn. Scribner Macmillan.
Book: My Mother’s Keeper: A daughter’s memoir of growing up in the shadow of schizophrenia (1997). Tara Elgin Holley. William Morrow Co.
Book: Recovered, Not Cured: A journey through schizophrenia (2005). Richard McLean. Allen and Unwin.
Book: The Center Cannot Hold: My journey through madness (2008). Elyn R. Saks. Hyperion.
Book: The Quiet Room: A journal out of the torment of madness (1996). Lori Schiller. Grand Central Publishing.
Book: Welcome Silence: My triumph over schizophrenia (2003). Carol North. CSS Publishing.
Web: National Alliance for the Mentally Ill. This is an excellent site for learning more about advocacy for individuals with major mental illnesses such as schizophrenia. http://www.nami.org/
Web: National Institute of Mental Health. This website has information on NIMH-funded schizophrenia research. http://www.nimh.nih.gov/health/topics/schizophrenia/index.shtml
Web: Schizophrenia Research Forum. This is an excellent website that contains a broad array of information about current research on schizophrenia. http://www.schizophreniaforum.org/
Discussion Questions
- Describe the major differences between the major psychotic disorders.
- How would one be able to tell when an individual is “delusional” versus having non-delusional beliefs that differ from the societal normal? How should cultural and sub-cultural variation been taken into account when assessing psychotic symptoms?
- Why are cognitive impairments important to understanding schizophrenia?
- Why has the inclusion of a new diagnosis (Attenuated Psychotic Syndrome) in Section III of the DSM-5 created controversy?
- What are some of the factors associated with increased risk for developing schizophrenia? If we know whether or not someone has these risk factors, how well can we tell whether they will develop schizophrenia?
- What brain changes are most consistent in schizophrenia?
- Do antipsychotic medications work well for all symptoms of schizophrenia? If not, which symptoms respond better to antipsychotic medications?
- Are there any treatments besides antipsychotic medications that help any of the symptoms of schizophrenia? If so, what are they?
References
APA. (2013). Diagnostic and statistical manual of mental disorders, Fifth Edition (5th ed.). Washington, DC: American Psychiatric Association.
Barch, D. M. (2020). Schizophrenia Spectrum Disorders. In R. Biswas-Diener & E. Diener (Eds), Noba textbook series: Psychology. Champaign, IL: DEF publishers. Retrieved from http://noba.to/5d98nsy4.
Barch, D. M., & Ceaser, A. E. (2012). Cognition in schizophrenia: Core psychological and neural mechanisms. Trends in Cognitive Science, 16, 27–34.
Barch, D. M., & Dowd, E. C. (2010). Goal representations and motivational drive in schizophrenia: The role of prefrontal-striatal interactions. Schizophrenia Bulletin, 36(5), 919–934. doi:sbq068 [pii] 10.1093/schbul/sbq068.
Barch, D. M., Carter, C. S., & Cohen, J. D. (2003). Context processing deficit in schizophrenia: Diagnostic specificity, 4-week course, and relationships to clinical symptoms. Journal of Abnormal Psychology, 112, 132–143.
Barch, D. M., Carter, C. S., Macdonald, A., Sabb, F. W., Noll, D. C., & Cohen, J. D. (1999). Prefrontal cortex and context processing in medication-naive first-episode patients with schizophrenia. Schizophrenia Research, 36(1–3), 217–218.
Batstra, L., & Frances, A. (2012). Diagnostic inflation: Causes and a suggested cure. The Journal of Nervous and Mental Disease, 200(6), 474–479. doi:10.1097/NMD.0b013e318257c4a2.
Boos, H. B., Aleman, A., Cahn, W., Pol, H. H., & Kahn, R. S. (2007). Brain volumes in relatives of patients with schizophrenia: A meta-analysis. Archives of General Psychiatry, 64(3), 297–304.
Bora, E., Fornito, A., Radua, J., Walterfang, M., Seal, M., Wood, S. J., . . . Pantelis, C. (2011). Neuroanatomical abnormalities in schizophrenia: A multimodal voxelwise meta-analysis and meta-regression analysis. Schizophrenia Research, 127(1–3), 46–57. doi:10.1016/j.schres.2010.12.020.
Bora, E., Yucel, M., & Pantelis, C. (2009a). Cognitive functioning in schizophrenia, schizoaffective disorder and affective psychoses: Meta-analytic study. The British Journal of Psychiatry: The Journal of Mental Science, 195(6), 475–482. doi:10.1192/bjp.bp.108.055731.
Bora, E., Yucel, M., & Pantelis, C. (2009b). Theory of mind impairment in schizophrenia: Meta-analysis. Schizophrenia Research, 109(1–3), 1–9. doi:10.1016/j.schres.2008.12.020.
Bourque, F., van der Ven, E., & Malla, A. (2011). A meta-analysis of the risk for psychotic disorders among first- and second-generation immigrants. Psychological Medicine, 41(5), 897–910. doi:10.1017/S0033291710001406.
Bowie, C. R., Leung, W. W., Reichenberg, A., McClure, M. M., Patterson, T. L., Heaton, R. K., & Harvey, P. D. (2008). Predicting schizophrenia patients’ real-world behavior with specific neuropsychological and functional capacity measures. Biological Psychiatry, 63(5), 505–511. doi:10.1016/j.biopsych.2007.05.022.
Bowie, C. R., Reichenberg, A., Patterson, T. L., Heaton, R. K., & Harvey, P. D. (2006). Determinants of real-world functional performance in schizophrenia subjects: Correlations with cognition, functional capacity, and symptoms. The American Journal of Psychiatry, 163(3), 418–425. doi:10.1176/appi.ajp.163.3.418.
Brans, R. G., van Haren, N. E., van Baal, G. C., Schnack, H. G., Kahn, R. S., & Hulshoff Pol, H. E. (2008). Heritability of changes in brain volume over time in twin pairs discordant for schizophrenia. Archives of General Psychiatry, 65(11), 1259–1268. doi:10.1001/archpsyc.65.11.1259.
Canadian Mental Health Association (2013). Psychosis. Retrieved from https://cmha.bc.ca/documents/psychosis-2/.
Cannon, M., Jones, P. B., & Murray, R. M. (2002). Obstetric complications and schizophrenia: Historical and meta-analytic review. The American Journal of Psychiatry, 159(7), 1080–1092.
Cannon, T. D., Cadenhead, K., Cornblatt, B., Woods, S. W., Addington, J., Walker, E., . . . Heinssen, R. (2008). Prediction of psychosis in youth at high clinical risk: A multisite longitudinal study in North America. Archives of General Psychiatry, 65(1), 28–37.
Carpenter, W. T., Jr., & Davis, J. M. (2012). Another view of the history of antipsychotic drug discovery and development. Molecular Psychiatry, 17(12), 1168–1173. doi:10.1038/mp.2012.121.
Casadio, P., Fernandes, C., Murray, R. M., & Di Forti, M. (2011). Cannabis use in young people: The risk for schizophrenia. Neuroscience & Biobehavioral Reviews. doi:S0149-7634(11)00073-X [pii] 10.1016/j.neubiorev.2011.04.007.
Cohen, A. S., & Minor, K. S. (2010). Emotional experience in patients with schizophrenia revisited: Meta-analysis of laboratory studies. Schizophrenia Bulletin, 36(1), 143–150. doi:10.1093/schbul/sbn061.
Corlett, P. R., Honey, G. D., & Fletcher, P. C. (2007). From prediction error to psychosis: Ketamine as a pharmacological model of delusions. Journal of Psychopharmacology, 21(3), 238–252. doi:21/3/238 [pii] 10.1177/0269881107077716.
Corlett, P. R., Honey, G. D., Aitken, M. R., Dickinson, A., Shanks, D. R., Absalom, A. R., . . . Fletcher, P. C. (2006). Frontal responses during learning predict vulnerability to the psychotogenic effects of ketamine: Linking cognition, brain activity, and psychosis. Archives of General Psychiatry, 63(6), 611–621. doi:63/6/611 [pii] 10.1001/archpsyc.63.6.611.
Corlett, P. R., Murray, G. K., Honey, G. D., Aitken, M. R., Shanks, D. R., Robbins, T. W., . . . Fletcher, P. C. (2007a). Disrupted prediction-error signal in psychosis: Evidence for an associative account of delusions. Brain: A Journal of Neurology, 130(Pt 9), 2387–2400. doi:10.1093/brain/awm173.
Corlett, P. R., Murray, G. K., Honey, G. D., Aitken, M. R., Shanks, D. R., Robbins, T. W., . . . Fletcher, P. C. (2007b). Disrupted prediction-error signal in psychosis: Evidence for an associative account of delusions. Brain, 130(Pt 9), 2387–2400. doi:awm173 [pii] 10.1093/brain/awm173.
Dominguez Mde, G., Viechtbauer, W., Simons, C. J., van Os, J., & Krabbendam, L. (2009). Are psychotic psychopathology and neurocognition orthogonal? A systematic review of their associations. Psychological Bulletin, 135(1), 157–171. doi:10.1037/a0014415.
Eack, S. M., Greenwald, D. P., Hogarty, S. S., & Keshavan, M. S. (2010). One-year durability of the effects of cognitive enhancement therapy on functional outcome in early schizophrenia. Schizophrenia Research, 120(1–3), 210–216. doi:S0920-9964(10)01222-3 [pii] 10.1016/j.schres.2010.03.042.
Eack, S. M., Greenwald, D. P., Hogarty, S. S., Cooley, S. J., DiBarry, A. L., Montrose, D. M., & Keshavan, M. S. (2009). Cognitive enhancement therapy for early-course schizophrenia: effects of a two-year randomized controlled trial. Psychiatr Serv, 60(11), 1468–1476. doi:60/11/1468 [pii] 10.1176/appi.ps.60.11.1468.
Eack, S. M., Hogarty, G. E., Cho, R. Y., Prasad, K. M., Greenwald, D. P., Hogarty, S. S., & Keshavan, M. S. (2010). Neuroprotective effects of cognitive enhancement therapy against gray matter loss in early schizophrenia: Results from a 2-year randomized controlled trial. Archives of General Psychiatry, 67(7), 674–682. doi:2010.63 [pii]10.1001/archgenpsychiatry.2010.63.
Eack, S. M., Pogue-Geile, M. F., Greenwald, D. P., Hogarty, S. S., & Keshavan, M. S. (2010). Mechanisms of functional improvement in a 2-year trial of cognitive enhancement therapy for early schizophrenia. Psychological Medicine, 1–9. doi:S0033291710001765 [pii] 10.1017/S0033291710001765.
Fett, A. K., Viechtbauer, W., Dominguez, M. D., Penn, D. L., van Os, J., & Krabbendam, L. (2011). The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: A meta-analysis. Neuroscience and Biobehavioral Reviews, 35(3), 573–588. doi:10.1016/j.neubiorev.2010.07.001.
Fioravanti, M., Carlone, O., Vitale, B., Cinti, M. E., & Clare, L. (2005). A meta-analysis of cognitive deficits in adults with a diagnosis of schizophrenia. Neuropsychology Review, 15(2), 73–95. doi:10.1007/s11065-005-6254-9.
Fletcher, P. C., Frith, C. D., Grasby, P. M., Shallice, T., Frackowiak, R. S. J., & Dolan, R. J. (1995). Brain systems for encoding and retrieval of auditory-verbal memory: An in vivo study in humans. Brain, 118, 401–416.
Forbes, N. F., Carrick, L. A., McIntosh, A. M., & Lawrie, S. M. (2009). Working memory in schizophrenia: A meta-analysis. Psychological Medicine, 39(6), 889–905. doi:10.1017/S0033291708004558.
Fusar-Poli, P., & Yung, A. R. (2012). Should attenuated psychosis syndrome be included in DSM-5? Lancet, 379(9816), 591–592. doi:10.1016/S0140-6736(11)61507-9.
Fusar-Poli, P., McGuire, P., & Borgwardt, S. (2012). Mapping prodromal psychosis: A critical review of neuroimaging studies. European Psychiatry: The Journal of the Association of European Psychiatrists, 27(3), 181–191. doi:10.1016/j.eurpsy.2011.06.006.
Fusar-Poli, P., Perez, J., Broome, M., Borgwardt, S., Placentino, A., Caverzasi, E., . . . McGuire, P. (2007). Neurofunctional correlates of vulnerability to psychosis: A systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews, 31(4), 465–484.
Gejman, P. V., Sanders, A. R., & Kendler, K. S. (2011). Genetics of schizophrenia: New findings and challenges. Annual Review of Genomics and Human Genetics. doi:10.1146/annurev-genom-082410-101459.
Gottesman, I. I., & Shields, J. (1967). A polygenic theory of schizophrenia. Proceedings of the National Academy of Sciences of the United States of America, 58(1), 199–205.
Green, M. F. (2006). Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. The Journal of Clinical Psychiatry, 67(9), 3–8; discussion 36–42.
Hafner, H., & an der Heiden, W. (1997). Epidemiology of schizophrenia. The Canadian Journal of Psychiatry, 42, 139-151.
Heckers, S., & Konradi, C. (2010). Hippocampal pathology in schizophrenia. Current Topics in Behavioral Neurosciences, 4, 529–553.
Hoe, M., Nakagami, E., Green, M. F., & Brekke, J. S. (2012). The causal relationships between neurocognition, social cognition, and functional outcome over time in schizophrenia: A latent difference score approach. Psychological Medicine, 1–13. doi:10.1017/S0033291712000578.
Hogarty, G. E., Greenwald, D. P., & Eack, S. M. (2006). Durability and mechanism of effects of cognitive enhancement therapy. Psychiatric Services, 57(12), 1751–1757. doi:57/12/1751 [pii] 10.1176/appi.ps.57.12.1751.
Jensen, J., & Kapur, S. (2009). Salience and psychosis: Moving from theory to practise. Psychological Medicine, 39(2), 197–198. doi:10.1017/S0033291708003899.
Jensen, J., Willeit, M., Zipursky, R. B., Savina, I., Smith, A. J., Menon, M., . . . Kapur, S. (2008). The formation of abnormal associations in schizophrenia: Neural and behavioral evidence. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 33(3), 473–479. doi:10.1038/sj.npp.1301437.
Kapur, S. (2003). Psychosis as a state of aberrant salience: A framework linking biology, phenomenology, and pharmacology in schizophrenia. American Journal of Psychiatry, 160(1), 13–23.
Kapur, S., Mizrahi, R., & Li, M. (2005). From dopamine to salience to psychosis—linking biology, pharmacology and phenomenology of psychosis. Schizophrenia Research, 79(1), 59–68. doi:10.1016/j.schres.2005.01.003.
Karlsgodt, K. H., van Erp, T. G., Poldrack, R. A., Bearden, C. E., Nuechterlein, K. H., & Cannon, T. D. (2008). Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biological Psychiatry, 63(5), 512–518.
Kerr, S. L., & Neale, J. M. (1993). Emotion perception in schizophrenia: Specific deficit or further evidence of generalized poor performance? Journal of Abnormal Psychology, 102(2), 312–318.
Kim, J. J., Kwon, J. S., Park, H. J., Youn, T., Kang, D. H., Kim, M. S., . . . Lee, M. C. (2003). Functional disconnection between the prefrontal and parietal cortices during working memory processing in schizophrenia: A [15O]H20 PET study. American Journal of Psychiatry, 160, 919–923.
Kim, Y., Zerwas, S., Trace, S. E., & Sullivan, P. F. (2011). Schizophrenia genetics: Where next? Schizophrenia Bulletin, 37(3), 456–463. doi:sbr031 [pii] 10.1093/schbul/sbr031.
Kohler, C. G., Walker, J. B., Martin, E. A., Healey, K. M., & Moberg, P. J. (2010). Facial emotion perception in schizophrenia: A meta-analytic review. Schizophrenia Bulletin, 36(5), 1009–1019. doi:10.1093/schbul/sbn192.
Kring, A. M., & Moran, E. K. (2008). Emotional response deficits in schizophrenia: Insights from affective science. Schizophrenia Bulletin, 34(5), 819–834.
Lieberman, J. A., Stroup, T. S., McEvoy, J. P., Swartz, M. S., Rosenheck, R. A., Perkins, D. O., . . . Hsiao, J. K. (2005). Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. The New England Journal of Medicine, 353(12), 1209–1223. doi:10.1056/NEJMoa051688.
Llerena, K., Strauss, G. P., & Cohen, A. S. (2012). Looking at the other side of the coin: A meta-analysis of self-reported emotional arousal in people with schizophrenia. Schizophrenia Research, 142(1–3), 65–70. doi:10.1016/j.schres.2012.09.005.
Lopez-Munoz, F., Alamo, C., Cuenca, E., Shen, W. W., Clervoy, P., & Rubio, G. (2005). History of the discovery and clinical introduction of chlorpromazine. Annals of Clinical Psychiatry: Official Journal of the American Academy of Clinical Psychiatrists, 17(3), 113–135.
Luzi, S., Morrison, P. D., Powell, J., di Forti, M., & Murray, R. M. (2008). What is the mechanism whereby cannabis use increases risk of psychosis? Neurotoxicity Research, 14(2–3), 105–112. doi:10.1007/BF03033802.
MacDonald, A. W., III, Thermenos, H. W., Barch, D. M., & Seidman, L. J. (2009). Imaging genetic liability to schizophrenia: Systematic review of FMRI studies of patients’ nonpsychotic relatives. Schizophrenia Bulletin, 35(6), 1142–1162.
Mamah, D., Mbwayo, A., Mutiso, V., Barch, D. M., Constantino, J. N., Nsofor, T., . . . Ndetei, D. M. (2012). A survey of psychosis risk symptoms in Kenya. Comprehensive Psychiatry, 53(5), 516–524. doi:10.1016/j.comppsych.2011.08.003.
March, D., Hatch, S. L., Morgan, C., Kirkbride, J. B., Bresnahan, M., Fearon, P., & Susser, E. (2008). Psychosis and place. Epidemiologic Reviews, 30, 84–100. doi:10.1093/epirev/mxn006.
Mesholam-Gately, R. I., Giuliano, A. J., Goff, K. P., Faraone, S. V., & Seidman, L. J. (2009). Neurocognition in first-episode schizophrenia: A meta-analytic review. Neuropsychology, 23(3), 315–336. doi:10.1037/a0014708.
Meyer-Lindenberg, A., Poline, J., Kohn, P. D., Holt, J. L., Egan, M. F., Weinberger, D. R., & Berman, K. F. (2001). Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. American Journal of Psychiatry, 158, 1809–1817.
Miller, B., Messias, E., Miettunen, J., Alaraisanen, A., Jarvelin, M. R., Koponen, H., . . . Kirkpatrick, B. (2011). Meta-analysis of paternal age and schizophrenia risk in male versus female offspring. Schizophrenia Bulletin, 37(5), 1039–1047. doi:10.1093/schbul/sbq011.
Minzenberg, M. J., Laird, A. R., Thelen, S., Carter, C. S., & Glahn, D. C. (2009). Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Archives of General Psychiatry, 66(8), 811–822. doi:10.1001/archgenpsychiatry.2009.91.
Murray, G. K., Corlett, P. R., Clark, L., Pessiglione, M., Blackwell, A. D., Honey, G., . . . Fletcher, P. C. (2008). Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Molecular Psychiatry, 13(3), 267–276.
Ndetei, D. M., Muriungi, S. K., Owoso, A., Mutiso, V. N., Mbwayo, A. W., Khasakhala, L. I., . . . Mamah, D. (2012). Prevalence and characteristics of psychotic-like experiences in Kenyan youth. Psychiatry Research, 196(2–3), 235–242. doi:10.1016/j.psychres.2011.12.053.
Nuechterlein, K. H., Subotnik, K. L., Green, M. F., Ventura, J., Asarnow, R. F., Gitlin, M. J., . . . Mintz, J. (2011). Neurocognitive predictors of work outcome in recent-onset schizophrenia. Schizophrenia Bulletin, 37 Suppl 2, S33–40. doi:10.1093/schbul/sbr084.
Olabi, B., Ellison-Wright, I., McIntosh, A. M., Wood, S. J., Bullmore, E., & Lawrie, S. M. (2011). Are there progressive brain changes in schizophrenia? A meta-analysis of structural magnetic resonance imaging studies. Biological Psychiatry, 70(1), 88–96. doi:10.1016/j.biopsych.2011.01.032.
Owen, M. J., Craddock, N., & O’Donovan, M. C. (2010). Suggestion of roles for both common and rare risk variants in genome-wide studies of schizophrenia. Archives of General Psychiatry, 67(7), 667–673. doi:10.1001/archgenpsychiatry.2010.69.
Preti, A., & Cella, M. (2010). Randomized-controlled trials in people at ultra high risk of psychosis: a review of treatment effectiveness. Schizophrenia Research, 123(1), 30–36. doi:10.1016/j.schres.2010.07.026.
Ragland, J. D., Laird, A. R., Ranganath, C., Blumenfeld, R. S., Gonzales, S. M., & Glahn, D. C. (2009). Prefrontal activation deficits during episodic memory in schizophrenia. American Journal of Psychiatry, 166(8), 863–874.
Repovs, G., & Barch, D. M. (2012). Working memory related brain network connectivity in individuals with schizophrenia and their siblings. Frontiers in Human Neuroscience, 6, 137. doi:10.3389/fnhum.2012.00137.
Rutter, M., Kim-Cohen, J., & Maughan, B. (2006). Continuities and discontinuities in psychopathology between childhood and adult life. Journal of Child Psychology and Psychiatry, and Aallied Disciplines, 47(3–4), 276–295. doi:10.1111/j.1469-7610.2006.01614.x.
Schizophrenia Society of Canada (2017-2018). Annual report. Retrieved from https://www.schizophrenia.ca/docs/SSC%20and%20SSCF%20Annual%20Report%20for%202017-2018.pdf.
Schlosser, R., Gesierich, T., Kaufmann, B., Vucurevic, G., Hunsche, S., Gawehn, J., & Stoeter, P. (2003). Altered effective connectivity during working memory performance in schizophrenia: A study with fMRI and structural equation modeling. Neuroimage, 19(3), 751–763.
Snitz, B. E., Macdonald, A. W., 3rd, & Carter, C. S. (2006). Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: A meta-analytic review of putative endophenotypes. Schizophrenia Bulletin, 32(1), 179–194.
Squire, L.R. (1992). Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychological Review, 99, 195–231.
Sullivan, P. F., Kendler, K. S., & Neale, M. C. (2003). Schizophrenia as a complex trait: Evidence from a meta-analysis of twin studies. Archives of General Psychiatry, 60(12), 1187–1192. doi:10.1001/archpsyc.60.12.1187.
Ventura, J., Hellemann, G. S., Thames, A. D., Koellner, V., & Nuechterlein, K. H. (2009). Symptoms as mediators of the relationship between neurocognition and functional outcome in schizophrenia: a meta-analysis. Schizophrenia Research, 113(2–3), 189–199. doi:10.1016/j.schres.2009.03.035.
Ventura, J., Thames, A. D., Wood, R. C., Guzik, L. H., & Hellemann, G. S. (2010). Disorganization and reality distortion in schizophrenia: a meta-analysis of the relationship between positive symptoms and neurocognitive deficits. Schizophrenia Research, 121(1–3), 1–14. doi:10.1016/j.schres.2010.05.033.
Ventura, J., Wood, R. C., & Hellemann, G. S. (2011). Symptom domains and neurocognitive functioning can help differentiate social cognitive processes in schizophrenia: A meta-analysis. Schizophrenia Bulletin. doi:10.1093/schbul/sbr067.
Verdoux, H., & van Os, J. (2002). Psychotic symptoms in non-clinical populations and the continuum of psychosis. Schizophrenia Research, 54(1–2), 59–65.
van Hooren, S., Versmissen, D., Janssen, I., Myin-Germeys, I., a Campo, J., Mengelers, R., . . . Krabbendam, L. (2008). Social cognition and neurocognition as independent domains in psychosis. Schizophrenia Research, 103(1–3), 257–265. doi:10.1016/j.schres.2008.02.022.

